
Thermodynamics and Combustion Laboratory
The Thermodynamics and Combustion Laboratory is headed by Professor Mohamad (Hameed) Metghalchi.
Research
The research concentrates on thermodynamics and combustion. The thermodynamic research involves equilibrium and non-equilibrium thermodynamics dealing with fundamental relations and determination of Onsager's coefficients for reacting gas mixtures.
The laboratory conducts both experimental and theoretical combustion research. The experimental work covers fundamental research in flame speed measurement and autoignition using constant volume vessel with high speed photograph. The theoretical kinetic studies is the implementation of Rate-Controlled Constrained-Equilibrium in complex chemical reacting system.
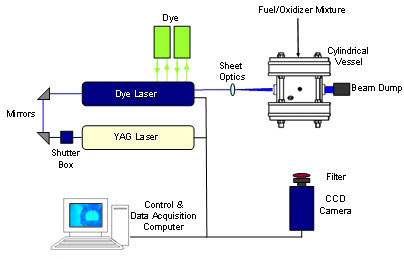
PLIF system
This is a Planar Laser Induced Fluorescence (PLIF) system for combustion diagnostics. It is an advanced system for concentration and temperature measurement. It is composed of several devices: Nd:YAG laser for pumping laser beam, Dye laser for changing the wavelength and frequency of the beam, CCD camera for detection of fluoresced data, and an Energy Monitor for normalization of laser beam.
This system can be used for atmospheric flames like flat flame and high temperature and pressure flames. A cylindrical vessel is located in this set up to measure the concentration of molecules within the high temperature and pressure flame. A 6 inch diameter central ignition vessel that can withstand pressures up to 425 atmosphere. It is equipped with piezoelectric pressure transducer and ionization probes.

This is the dye laser.

Camera and cylindrical vessel.
Modeling Combustion Using the Rate-Controlled Constrained-Equilibrium (RCCE) Method
Modeling of a non-equilibrium combustion process involves the solution of large systems of differential equations with as many equations as species present during the process.
The process of chemical reaction and combustion is complicated since it may be governed by hundreds, sometimes thousands of microscopic rate processes. Integration of these equations simultaneously becomes more difficult with the complexity of the combustible. In order to reduce the size of these systems of equations, the Rate-Controlled Constrained-Equilibrium method (RCCE) is used within our group to model non-equilibrium combustion processes.
This method is based on the Second Law of Thermodynamics, assuming that the evolution of a complex system can be described by a small number of rate-controlling reactions which impose slowly changing constraints on all allowed states of the system, therefore a non-equilibrium system will relax to its final equilibrium state through a sequence of rate controlled constrained equilibrium states.
Oxidation induction times and concentration of species during a combustion process are found in a less complicated way with this method, as equations for constraints rather than for species determine the composition and evolution of the system. The time evolution of the system can be reduced since the number of constraints is much smaller than the number of species present, so the number of equations to solve.
The RCCE method has been successfully applied to the stoichiometric combustion of mono-carbon fuels. Results of using 8, 9, 10 and 11 constraints compared very well to those of the detailed calculations at all conditions for the cases of formaldehyde (H2CO), methanol (CH3OH) and methane (CH4). For these systems, ignition delay times and major species concentrations were within 5% of the values given by detailed calculations, and computational saving times up to 50% have been met.
Currently our group is working on the application of this technique to heavier hydrocarbons, as well as to broaden the range of applications for the mono-carbon combustion modeling.